dot diagram for sulfur|sulfur electron dot diagram : Pilipinas Draw a valid electron dot structure for each of the given elements. Since fluorine is found in Group 7A of the periodic table, it contains 7 valence electrons. . webDireto. SIC em Direto. Guia TV. Mais populares. 0. Previous "A PGR deve ser substituída por uma pessoa com autoridade e capacidade de liderança" Análise de Marques Mendes .
0 · what is a lewis symbol
1 · sulfur electron dot diagram
2 · lewis electron dot symbol
3 · lewis electron dot diagram
4 · lewis dot symbol of sulfur
5 · lewis dot diagram of sulfur
6 · how to draw dot structures
7 · drawing electron dot diagrams
8 · More
7 de out. de 2023 · Bem-vindo ao site Giga Bicho, aqui você confere o resultado do Deu no Poste de hoje, sábado dia 07 de outubro, a equipe do site Giga Bicho informa os resultados do Deu no Poste do jogo do bicho ao vivo.. No dia de hoje, sábado o Deu no Poste possui cinco sorteios, o primeiro resultado é informado a partir das 11 horas, os números são .
dot diagram for sulfur*******A dot diagram sulfur is a visual representation of the valence electrons in a sulfur atom. It uses dots or crosses to indicate the number of valence electrons. This diagram is .
Draw a valid electron dot structure for each of the given elements. Since fluorine is found in Group 7A of the periodic table, it contains 7 valence electrons. .
Learn how to draw Lewis symbols for neutral atoms and ions using dots to represent valence electrons. See examples of sulfur and other elements in the third period of the periodic table.
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.
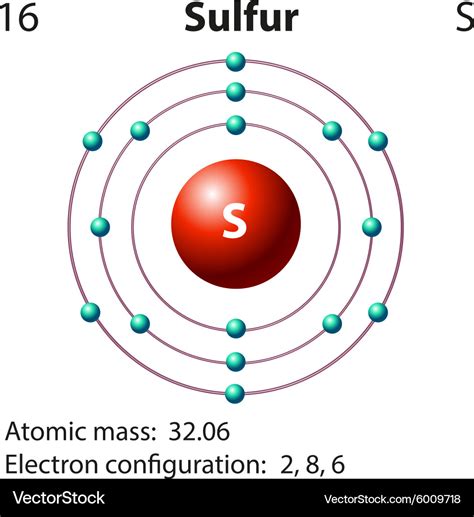
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses . A step-by-step explanation of how to draw the S2- Lewis Dot Structure.For the S2- Lewis structure use the periodic table to find the total number of valence .In summary, drawing a Lewis Dot Diagram for sulfur involves placing six valence electrons around the sulfur symbol as pairs of dots. This diagram helps in .
We draw the dot structure in the exact same manner, and then calculate the formal charges for the atoms in the molecule. Remember that formal charge is calculated by taking the # of .
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .

To create the dot diagram for sulfur, we start by placing one dot on each side of the symbol. This accounts for 4 electrons. We then pair up the remaining 2 electrons on one side, resulting in a total of 6 valence electrons represented by dots. The dot diagram for sulfur can be visually represented as follows: S.:. This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle,.Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.
More on the dot structure for sulfur dioxide. VSEPR for 4 electron clouds. VSEPR for 5 electron clouds (part 1) VSEPR for 5 electron clouds (part 2) . So now we have CH2O, which is the .dot diagram for sulfur sulfur electron dot diagram S : . The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). it has 6. sulfur has six .Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. Video: Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to . The Lewis structure of an atom represents the nucleus together with the valence electrons of that particular atom. As the electrons are represented in the form of dots, this model is also known as the electron dot structure. It is a visual representation of the atomic structure. The Lewis model of the sulfur atom is represented as follows:dot diagram for sulfurhe says the bond angle for sulfur dioxide (bent) is 120 degrees, the same as for a trigonal planar structure. In my chemistry course, however, my teacher explained that the lone electron pair on the sulfur atom will exert a stronger repulsive force and thus push the oxygen atoms further away, resulting in a smaller bond angle of about 115 degrees.A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.SO2 Lewis Structure Step-by-Step Guide. To draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 valence electrons, while each oxygen atom in Group 6 contributes 6 valence electrons.
Draw the lewis dot structure for chloroform, CHCl3. In chloroform's molecular formula, the carbon is first, and there is only one atom of carbon. So, carbon is the central atom. . The lewis structure of sulfur dioxide has sulfur double bonded to both oxygens. The sulfur also has a lone pair of electrons.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the .A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how many valence electrons Sulfur has..A dot diagram sulfur is a visual representation of the valence electrons in a sulfur atom. It uses dots or crosses to indicate the number of valence electrons. This diagram is useful for understanding the bonding and chemical properties of sulfur. Draw a valid electron dot structure for each of the given elements. Since fluorine is found in Group 7A of the periodic table, it contains 7 valence electrons. Sulfur, which is located in Group 6A, has 6 valence electrons. A chemically-correct electron dot structure for each of these elements is shown below. Explain the meaning of an electron dot diagram. Draw electron dot diagrams for given elements. Describe the patterns of electron dot diagrams in the Periodic Table.A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals .sulfur electron dot diagramA Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals .
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
5,137 likes, 79 comments - renatoalbani on February 25, 2024: "Derrubei em 30s igual o popó ontem! kkkkk Querem video pro youtube amanhã? "
dot diagram for sulfur|sulfur electron dot diagram